Years of Service
The Dayton Clinical Oncology Program has been serving Dayton and surrounding areas since 1983. Dayton was one of the initial members of the National Cancer Institute (NCI) Community Clinical Oncology Program.
Our Mission...
To reduce cancer incidence and mortality through improved treatment and prevention by offering national state-of-the-art cancer research to the local communities.
The Dayton Clinical Oncology Program has been serving Dayton and surrounding areas since 1983. Dayton was one of the initial members of the National Cancer Institute (NCI) Community Clinical Oncology Program.
Since 1983 Dayton Clinical Oncology Program has participated in over 1500 clinical trials, helping to find better ways to prevent and treat cancer.
Over 8000 patients have participated in clinical trials at our locations. Every current standard treatment exists because patients joined clinical trials.
We are a regional non-profit cancer research consortium serving the Miami Valley area as well as Cincinnati, Findlay, Greenville, Middletown, Troy and Youngstown, Ohio; Northern Kentucky and Eastern Indiana.
Dayton Clinical Oncology Program, in cooperation with our participating area hospitals, universities and physicians, provides local access to national state-of-the-art cancer clinical trials through the National Cancer Institute and its Community Oncology Research Programs (NCORP).
Dayton Clinical Oncology Program, incorporated in 1983, became one of the initial members of the community oncology program. Today, we continue to offer NCI- funded clinical trials, as well as Foundation funded clinical trials for cancer.
When Mary Ontko went to nursing school, one specialty stood out among all others.
“I did an oncology rotation and I loved it,” she said. “I thought, ‘This is where I want to be.’”
That exposure has resulted in decades of working for and with cancer patients, most recently at the Dayton Clinical Oncology Program, where she stepped down in November after serving as its president and chief executive officer since 2014.
Her achievements there earned her the 2022 Dorothy Coleman Outstanding Administrator Award, given out annually by the National Cancer Institute’s Community Oncology Research Program.
“Just for her to be recognized by the National Cancer Institute was something that was unbelievable. I was so proud,” said Michelle Kinney, who nominated Ontko as a Dayton Daily News Community Gem on behalf of the Dayton NCORP staff.
The organization offers NCI-funded clinical trials to cancer patients in the Miami Valley, Cincinnati, Findlay and Youngstown areas, in addition to Northern Kentucky and Eastern Indiana.

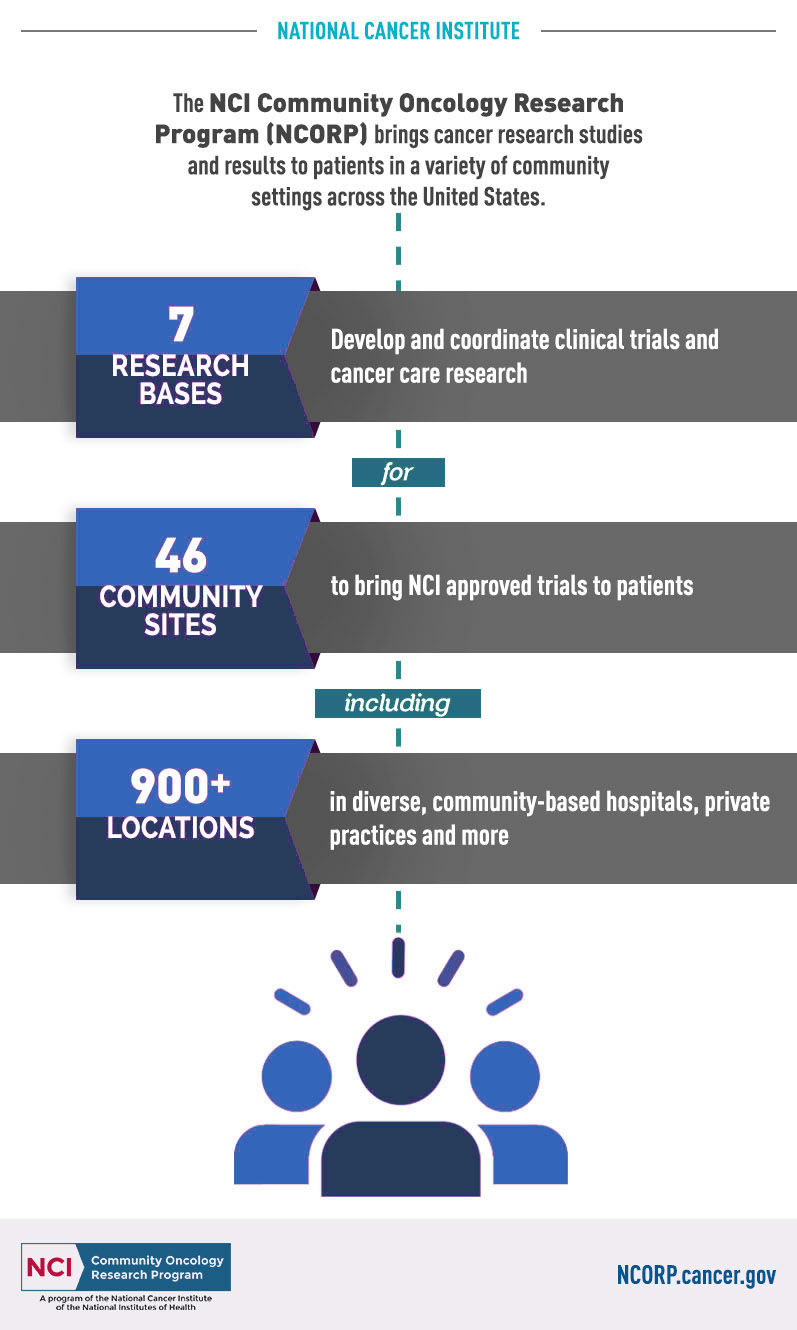
The NCI Community Oncology Research Program (NCORP) is a national NCI-supported network that brings cancer treatment and cancer control and prevention clinical trials to people in their communities, while also looking at different aspects of cancer care with cancer care delivery research (CCDR).
In the early 1980's, the Federal Government found that over 85% of cancer patients were being treated in their local communities rather than at large cancer treatment centers. They initiated funding for the Community Clinical Oncology Program through the National Cancer Institute (NCI), one of the branches of the National Institutes of Health. Dayton Clinical Oncology Program was one of those original community sites, and has been providing access to national oncology clinical trials since 1983. In 2014, Dayton Clinical Oncology Program was awarded a five-year grant from the NCI, and is one of 46 Community Sites in the United States to transition from an NCI Community Clinical Oncology Program (CCOP) to an NCI Community Oncology Research Program (NCORP).
NCORP is comprised of 7 Research Bases and 46 Community Sites. The NCORP Research Bases are hubs for the network that design and spearhead the conduct of multi-center clinical trials and CCDR, and provide overall administration, data management, scientific and statistical leadership, operational processes and personnel, and regulatory compliance.
The NCORP Community Sites accrue patients and participants to NCI-approved cancer clinical trials and research studies. The Sites are consortia of researchers, public hospitals, physician practices, academic medical centers, and other groups that provide healthcare services in communities across the U.S.
If you are interested in a clinical trial or would like more information about a clinical trial, speak with your oncologist, surgeon, family practice physician, or contact Dayton Clinical Oncology Program and we will be glad to assist you.
We maintain an active list of approximately 100 clinical trials for the treatment of cancers of the brain, breast, gastrointestinal, genitourinary, head and neck, leukemia, lung, lymphoma, melanoma, myeloma, sarcoma, and other sites, and for cancer prevention and cancer control and cancer care delivery research (CCDR).
The policy and procedures outlined are intended to meet the most recent requirements published by the federal government regarding revised Financial Conflict of Interest (FCOI) Regulation, Promoting Objectivity in Research on August 25, 2011 (42 CFR Part 50 Subpart F and 45 CFR Part 94).
Dayton Clinical Oncology Program is committed to ensuring that our website is accessible to everyone, including people with disabilities. We strive to adhere to the Web Content Accessibility Guidelines (WCAG) 2.1 Level AA (or WCAG 2.2 AA), which outline best practices for creating inclusive digital experiences.
If you encounter accessibility barriers on our website or need content in an alternative format, we welcome your feedback and will assist as quickly as possible.
Contact Us:
Email: ClinicalTrials@daytonncorp.org
Phone: 937-528-5900